The physical phenomenon of latent heat has been understood for centuries as one of the key components of the study of thermal energy behavior. More recently, the importance of latent heat has taken a new meaning as a facilitator of crucial operations for developing a sustainable heating industry. Key to this is the development of new ways to overcome the challenges to heating storage.
But what exactly is latent heat, what are the two types of latent heat and how is it being used to enhance heating systems? Let’s take a look.
What is latent heat?
Latent heat is a term used to refer to the amount of heat energy that is absorbed or released by a substance during a phase change, taking place without a change in temperature. As such, it’s present during phenomena such as melting, freezing, vaporization, or condensation, representing the energy that is required to break or form the intermolecular forces holding the particles together in a substance.
The use of the term “latent” (a synonym to “hidden”) describes how this heat energy is hidden within the substance’s molecular structure during the phase transition. When the phase change happens, its internal energy changes even though its temperature remains constant.
This is due to the changes in the arrangement and movement of molecules during a phase change, so that intermolecular forces, bonds, between molecules are either broken or formed. This energy change is known as latent heat.
All in all, the concept of latent heat is rooted in the principle of conservation of energy. This means energy absorbed or released as latent heat is balanced by an equal amount of energy gained or lost by the surrounding environment. The result is temperature regulation during phase transitions.
Latent heat is expressed as an amount of energy per unit mass (e.g., joules per kilogram or calories per gram).
The formula for latent heat is:
Q = m x L
In which, Q is the amount of heat that is absorbed or released, m is the mass of the substance and L is the Specific Latent.
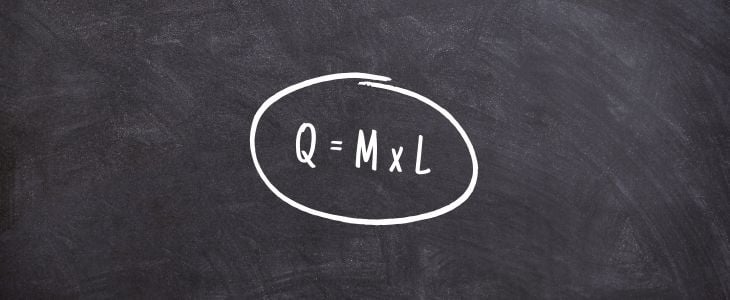
What are the two types of latent heat?
1. Latent heat of fusion
The latent heat of fusion refers to the amount of heat energy required to change a substance from a solid to a liquid state (melting) or vice versa (freezing) at a constant temperature.
On the one hand, latent heat of fusion is absorbed when a solid melts. The heat energy is absorbed by the particles, which see their kinetic energy increased and causes them to vibrate more rapidly. As the temperature reaches melting point, the additional heat energy supplied is used to weaken the intermolecular forces that hold the particles in a fixed arrangement. Once these forces are overcome, the particles gain enough energy to transition into a liquid state.
On the other hand, latent heat of fusion is released when a liquid freezes. That is, when a liquid substance is cooled, the heat energy is released from the particles as the intermolecular forces become stronger, causing the liquid to solidify. This released heat energy is equal to the latent heat of fusion.
2. Latent heat of vaporization
Latent heat of vaporization (or condensation) is the amount of heat energy required to change a substance from a liquid to a gas state (vaporization or evaporation) or vice versa (condensation) at a constant temperature.
Similarly to the process explained above, the latent heat of vaporization is absorbed when a liquid vaporizes and released when a gas condenses.
The applications of latent heat
As we’ve mentioned above, the potential of latent heat for the heating industry is bringing this phenomenon to the forefront. As both private and public operators and investors seek to develop sustainable heating technologies, latent heat opens the door to increased efficiencies and optimizations.
At the ready, it’s being utilized in heating and cooling systems such as air conditioners and refrigerators. Certain systems use the vaporization of a refrigerant to absorb latent heat from the surrounding environment, causing cooling. Conversely, when the refrigerant condenses back into a liquid, latent heat can be released to provide heating or maintain lower temperatures.
But latent heat is also crucial for state-of-the-art thermal energy storage systems. These structures are enabling the implementation of renewable energy sources for heating, and they are doing so through phase change materials.

PCMs are substances with high latent heat capacities, so that they can absorb and release large amounts of heat energy during their phase transitions. For instance, some common PCM materials include paraffin wax, salt hydrates and certain organic compounds. These substances are being employed as efficient thermal energy storage mediums in a variety of applications, from renewable energy storage, to building insulation, and temperature regulation systems.
In addition to latent heat storage, there are at least two other formulas being explored in thermal storage technology:
- Thermochemical storage, which works by employing reversible chemical reactions that involve heat absorption or release.
- Sensible heat storage, which relies on changing the temperature of a storage medium.
However, energy stored in latent heat transitions is higher than that of sensible heat systems, presenting obvious advantages for certain projects. Such is the case of water, which is able to provide approximately 80 times more energy by following latent heat processes than through rising its temperature from 1 to 2 degrees.
Innovation in sustainable heating systems
At ARANER, we’re at the forefront of cutting-edge heating systems that aim for maximum efficiency and sustainability. As such, we develop thermal storage tanks as well as district heating initiatives that are changing the way sustainable heating is done.
Get in touch with us and discover how we can help you find the right system for your project.










